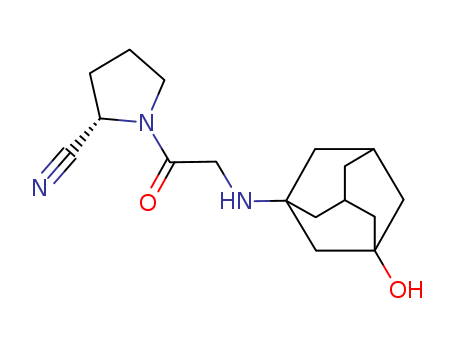
Vildagliptin 274901-16-5 CAS NO.274901-16-5
- Min.Order: 1 Kilogram
- Payment Terms: L/C,T/T
- Product Details
Keywords
- Vildagliptin
- 274901-16-5
- C17H25N3O2
Quick Details
- ProName: Vildagliptin 274901-16-5
- CasNo: 274901-16-5
- Molecular Formula: C17H25N3O2
- Appearance: white powder
- Application: Vildagliptin, previously identified as...
- DeliveryTime: spot goods
- PackAge: 25kg/tub
- Port: shanghai
- ProductionCapacity: 300 Kilogram/Month
- Purity: 99.5%
- Storage: Room temperature preservation
- Transportation: By air or by shipment
- LimitNum: 1 Kilogram
- Related Substances: pass
- Residue on Ignition: pass
- Heavy Metal: pass
- Valid Period: 24 moths
Superiority
Vildagliptin, previously identified as LAF237, is a new oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucaon release by the alpha cells of the islets of Langerhans in the pancreas. It is currently in clinical trials in the U.S. and has been shown to reduce hyperglycemia in type 2 diabetes mellitus. While the drug is still not approved for use in the US, it was approved in Feb 2008 by European Medicines Agency for use within the EU and is listed on the Australian PBS with certain restrictions.
Details
|
Vildagliptin, previously identified as LAF237, is a new oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucaon release by the alpha cells of the islets of Langerhans in the pancreas. It is currently in clinical trials in the U.S. and has been shown to reduce hyperglycemia in type 2 diabetes mellitus. While the drug is still not approved for use in the US, it was approved in Feb 2008 by European Medicines Agency for use within the EU and is listed on the Australian PBS with certain restrictions. |
|
| Structure |
|---|





